Abstract
Introduction: Hypomethylating agents (HMAs) are used for the treatment of patients with myelodysplastic syndromes (MDS). Two HMAs, decitabine and azacitidine, are currently available for such treatment. Numerous studies have analyzed the clinical efficacy of HMAs in patients with MDS; however, reports directly comparing decitabine and azacitidine in patients with lower-risk (low and intermediate-1) MDS are limited. The clinical efficacy of standard-dose HMA treatment in lower-risk MDS remains controversial.
Patients and methods: The Korea University MDS registry is a longitudinal cohort that contains data on 452 patients consecutively diagnosed with MDS from October 2006 to December 2017 in Korea University Medical Center (Korea University Anam, Guro, and Ansan Hospital). In the Korea University MDS registry, 357 patients were classified as having lower-risk MDS. Among them, 115 patients were treated with HMA (decitabine or azacitidine); 111 patients were eligible for the study. We compared treatment responses, survival outcomes, and adverse events between standard-dose decitabine (20 mg/m2 daily for 5 days every 4 weeks) and azacitidine (75 mg/m2 daily for 7 days every 4 weeks) in lower-risk MDS patients. Treatment responses were assessed according to the modified 2006 International Working Group response criteria. Patients who were evaluated received at least one cycle of HMA therapy. The overall response rate (ORR) included complete remission (CR), partial remission, marrow CR, and hematologic improvement. Progression-free survival (PFS) was measured from the time of treatment initiation until disease progression or death from MDS.
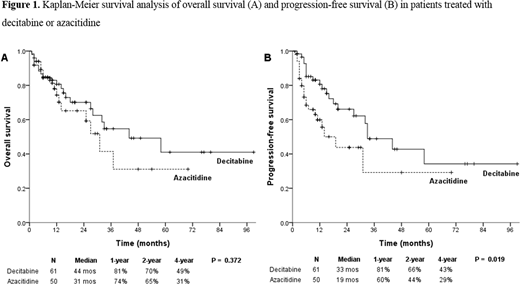
Results: The CR rates were 16.4% (10/61) in the decitabine group and 6.0% (3/50) in the azacitidine group with borderline significance (P = .090). The ORRs were 67.2% (41/61) and 44.0% (22/50) for decitabine and azacitidine, respectively (P = .014). The erythroid responses for decitabine and azacitidine were 68.3% (41/60) and 44.2% (19/43), respectively (P = .014). In the multivariable analysis, treatment with decitabine (hazard ratio [HR] 2.553; 95% confidence interval [CI] 1.116-5.840; P = .026), hemoglobin (Hb) concentration of <8 g/dL (HR 3.073; 95% CI 1.340-7.048; P = .008), and ≥5% BM blasts (HR 3.739; 95% CI 1.102-12.683; P = .034) were significantly associated with higher ORRs. The median progression-free survival was significantly better in patients treated with decitabine than in those treated with azacitidine (33 vs. 19 months; P = .019). There were no significant differences in the event-free survival and in the overall survival between the two HMAs. In the multivariable analysis, treatment with decitabine (HR 0.496; 95% CI 0.257-0.957; P = .037) and achievement of CR (HR 0.122; 95% CI 0.015-0.993; P = .049) were significant prognostic factors for better survival, whereas ANC below 0.8 × 109/L (HR 1.905; 95% CI 1.032-3.515; P = .039) was a significant prognostic factor for poor prognosis. The poor cytogenetic risk group, as classified by International Prognostic Scoring System (HR 2.136; 95% CI 0.992-4.556; P = .052), also affected the survival unfavorably with borderline significance. There were no significant differences in grade 3 or higher hematologic adverse events between the two HMAs.
Conclusions: The standard-dose decitabine therapy showed significantly better ORR, erythroid response, and longer PFS than did standard-dose azacitidine in patients with lower-risk MDS. The frequencies of hematologic adverse events did not differ between the patients who received decitabine and those who received azacitidine.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal